Preparation of Bases
Preparation of Bases: Overview
In this topic, we will study the different methods of preparation of bases. We will learn about various substances from which we can prepare bases. It also enhances our knowledge regarding the characteristics and properties of bases.
Important Questions on Preparation of Bases
Which of the following oxide turns red litmus into blue?
Which one of the following oxide is insoluble in water?
The nature of solution obtained by dissolving soluble metal oxide in water is:
Potassium oxide reacts with water to form
Write the balanced chemical equation for reaction of potassium oxide with water.
A student took solid quick lime in a china dish and added a small amount of water. He heard
Complete the following equation:
_____.
Basic oxides dissolve in water to give a alkali.
Give the preparation of the base, .
Observe the given figure carefully, which represents the decomposition of brine solution by passing electricity.
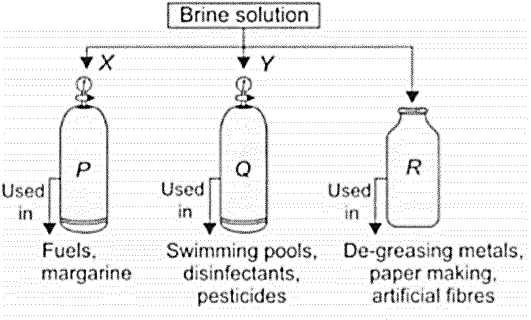
| (A) | |||||
| (B) | |||||
| (C) | |||||
| (D) |
Write chemical equation for the reaction of water with the following compound.
Magnesium oxide
Complete the following reaction.
