Isomerism
Isomerism: Overview
This Topic covers sub-topics such as Isomerism, Homologous Series, Structural Isomerism, Homologous Series of Alkenes, Homologous Series of Alkanes, Homologous Series of Alkynes, Structural Isomers of C6H14 and, Properties of Homologous Series
Important Questions on Isomerism
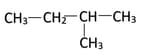
What is the IUPAC name of the given structural isomer of hexane:
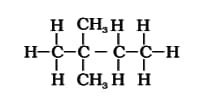
The number of structural isomers of hexane is:
Which one is the structural isomer of ?
Number of sigma bonds in neo-pentane is .
Which one is not the structural isomer of pentane?
Geometrical isomerism is possible with alkenes.
Choose the correct molecular formula and structural formula of an alkene having five carbon atoms:
The first member of alkane that shows structural isomerism is:
For pentane total of three structural isomers are possible.
Identify the structural isomers of pentane.
Isomers are the chemical compound that has the same _____ formula but a different structural arrangement.
To complete the following flowchart, the compounds needed to be filled in the blanks respectively are:
The molecular formula of the compounds in a homologous series are . The suitable general formula for the given compounds is:
Define the homologous series of carboxylic acids.
A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a _____ series.
Which of the following hydrocarbons are alkanes?