The Modern Periodic Table
The Modern Periodic Table: Overview
This Topic covers sub-topics such as Halogens, The Modern Periodic Table, Trends in Modern Periodic Table, Alkali Metals and Alkaline Earth Metals, Position of Elements in Modern Periodic Table and, Trends of Electronegativity in Periodic Table
Important Questions on The Modern Periodic Table
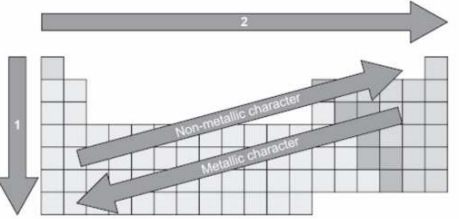
The picture shows an incomplete periodic table.
Arrow 1 and arrow 2 indicate increase or decrease in chemical properties.
The family of elements having seven electrons in the outermost shell is:
An element has configuration . It belongs to,
Identify the metals from the following elements that are present in the Modern periodic table
The modern periodic table is based on increasing atomic mass.
According to modern periodic law, the properties of the elements are in periodic function of their _____.
The position of 3 elements A, B and C in the Periodic Table are shown below.
Group 16 | Group 17
___________| __________
__________ | ____A_____
__________ | __________
____B_____|___ C______
Which of the following statements are correct ?
An element belongs to group 17. It is present in the third period and its atomic number is 17. The atomic number of the element belonging to the same group and present in the fifth period will be
An element X combines with hydrogen to form a compound XH3. The element X is placed on the right side of the Periodic Table. Which of the following statements are correct for element X?
The correct order of increasing acidic nature of SO2, SiO2, P2O3 and Al2O3 is
Which of the following statement(s) about the Modern Periodic Table are incorrect?
An element Y has a total of three shell, with six electrons in its valence shell.The atomic number of this element is
An element is placed in the second group and 4th period of the periodic table burns in the presence of oxygen to form basic oxide. The element is_____.
The tendency to gain electrons _____on moving down a group in the Periodic table.
The atomic radii of three elements A, B and C of a periodic table are 186 pm, 104 pm and 143 pm respectively. The increasing order of atomic numbers in the period is
Fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) were placed in group 17 of the Periodic Table. The name of this family is_____.
Elements of group 18 are called_____.
Which of the following elements would lose electron easily?
Which one of the following elements exhibit the maximum number of valence electrons?
The atomic number of elements A, B, C, D and E are 9, 11, 17, 12 and 13 respectively.
Which of the following pairs of elements belong to the same group?
