Thermometry
Thermometry: Overview
This topic covers concepts, such as Thermal Energy, Temperature, Thermometry, Thermometers, Mercury Thermometers, Gas Thermometers, Linear Thermodynamic Properties of Matter, Temperature Scales, Freezing Point of Water, Boiling Point of Water, Romer Scale, Celsius Scale, Fahrenheit Scale, Kelvin Scale, Absolute Zero Temperature, Fahrenheit Temperature Versus Celsius Temperature & Temperature Conversion Relations etc.
Important Questions on Thermometry
A solid cube and a solid sphere of identical material and equal masses are heated to the same temperature and left to cool in the same surroundings. Then
Equal masses of X, Y, and Z solids are heated
delivered at a constant rate by identical heaters.
If ex, CY and CZ and the specific heat of X, Y
and Z in its liquid state which was solid is t
= 0 then which of the following relation satisfy the
Temp-Time graph representing the processes
these substances underwent.
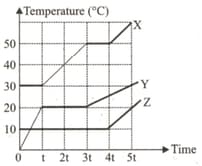
Table shows the mass and final temperature of three solids whose initial temperature is T and whom heat has been supplied by same heater at the constant rate for some time, m seconds.
Matter Mass Final
TemperatureX m 2T Y 3m 2T Z 2m 3T Which of the following statements is correct? (No process change and evaporation occurs.)
Which of the following is constant in a diathermic boundary?
The temperature which has same numerical value on Celsius and Fahrenheit scale is:
