Dipole Moment
Dipole Moment: Overview
This topic covers concepts such as dipole moment, application of dipole moment to determine geometry of molecules, and dipole moment comparison for NH3 and NF3.
Important Questions on Dipole Moment
Among the following, the molecule with the highest dipole moment is
Which of the following compounds, hydrocarbons, has the lowest dipole moment?
Among the following chloro-compound having the lowest dipole moment is:
Which one of the following pairs is an example of polar molecular solids?
The pair from the following pairs having both compounds with net non-zero dipole moment is
If the dipole moment of molecule 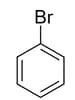 is , then for given molecule(s), correct options is/are
is , then for given molecule(s), correct options is/are
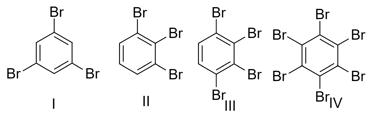
In which of the following pair trans isomer has higher dipole moment than cis isomer ?
Identify the correct order of dipole moment
Define dipole moment and explain with an example.
Select the structures which have a permanent dipole moment ?
Out of given ten molecules total molecules which have dipole moment zero is
Which of the following molecules is polar
Give an example to show dipole-dipole forces.
Which out of and has higher dipole moment?
One among the following posses zero dipole moment
Which of the following molecule does not have a net dipole moment?
Choose the correct ordering for the dipole moments of the following molecules:
If is the magnitude of charge and is the distance between the centres of positive and negative charges then dipole moment is given by
Identify the correct statements from the following
(a) The dipole moment of and is zero.
(b) The dipole moment of is higher than the dipole moment of .
(c) The dipole moment of is lower than the dipole moment of .
