Valence Shell Electron Pair Repulsion Theory
Valence Shell Electron Pair Repulsion Theory: Overview
This topic covers concepts such as Valence Shell Electron Pair Repulsion Theory, Geometry of Molecules Containing Two Bond Pairs and One Lone Pair, Geometry of Molecules Containing Three Bond Pairs and One Lone Pair, etc.
Important Questions on Valence Shell Electron Pair Repulsion Theory
Given below are two statements :
Statement I : and both possess V-shaped structure
Statement II : The bond angle of is less than that of .
In the light of the above statements, choose the most appropriate answer from the options given below:
Statement 1: both have bent structures.
Statement 2: have less angle than .
Which of the following has the maximum number of lone pairs on central atom ?
Match the following :
| (a) | (p) Linear |
| (b) | (q) Tetrahedral |
| (c) | (r) Bent |
| (d) | (s) See-saw |
How many of above molecules are having only two lone pair of electrons.
How many of the following are bent in shape?
.
The sum of number of lone pairs in central atom in is:
Which of the following have square pyramidal structure?
How many lone pairs are there in the structure CO2?
The correct order of the bond angles in and is
Which of the following is not pyramidal species?
Number of Bond angle of in is:
Consider the following figures and select the incorrect order of bond angle?
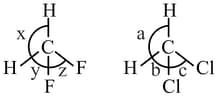
Which combination(s) given below is/are correct?
Comment on the statement: Molecules having two bond pairs and one lone pair has a trigonal planar shape.
Molecules having two bond pairs and one lone pair has trigonal planar shape.
Why do the lone pair and lone pair repulsion higher than bond pair repulsions?
What is the geometry of methane according to VSEPR theory.
Why do the lone pair and lone pair repulsion higher than bond pair repulsions?
How many bonds are present in product when is strongly heated (above )?
