General Properties of d-Block Elements
General Properties of d-Block Elements: Overview
This Topic covers sub-topics such as Coinage Metals, Lanthanoid Contraction, Standard Electrode Potential of d-Block Elements, Physical Properties of d-Block Elements and, Catalytic Properties of d-Block Elements
Important Questions on General Properties of d-Block Elements
Which one of the following is an amphoteric oxide?
The graph below shows the observed standard electrode potential of some transition elements.
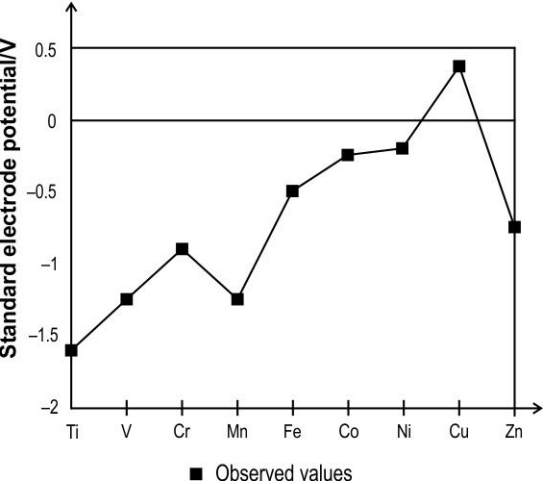
Which of the following reactions can be predicted based on the graph above?
Discuss the inert pair effect in d block elements.
Define coinage metals.
Complete the following catalytic mechanism,
Write the catalytic mechanism of the reaction between iodide and persulphate ions.
When iron catalyses the reaction between iodide and persulphate ions. Iron acts as a catalyst.
Which of the following represents the incorrect order of property mentioned?
Metals that are usually used as catalysts belong to
Which of the two have an almost similar size?
Which of the following oxide shows electrical properties like metals?
Transition elements show variable state oxidation because there is a very large energy difference in between and orbitals.
The metal that is present in brass, bronze, and German silver is :
Lanthanoid contraction is the gradual decrease in the atomic and ionic size of Lanthanoids with a decrease in atomic number.
Lanthanoid contraction is the gradual decrease in the atomic and ionic size of Lanthanoids with a decrease in atomic number.
Predict the correct order of the metallic character among the following:
Which of the following has the highest electrical conductance?
Sacandium and Yttrium have similar electrical conductance.
Copper is a good electrical _____.
Generally the d-block elements have
