Chemical Properties of Group 18 Elements
Chemical Properties of Group 18 Elements: Overview
This Topic covers sub-topics such as Uses of Noble Gases, Chemical Properties of Noble Gases, Compounds of Noble Gases, Xenon-Oxygen Compounds, Xenon - Fluorine Compounds, Preparation of Xenon-oxygen Compounds and, Structures of Xenon - Fluorine Compounds
Important Questions on Chemical Properties of Group 18 Elements
The product obtained on complete hydrolysis of and would be:
Number of lone pairs of electrons on atoms in and molecules are respectively.
The formation of is the basis for the formation of xenon fluorides. This is because
Partial hydrolysis of gives
Among the following, the relative acidity based on oxide acceptor nature of Xenon fluorides, Oxyflourides and oxides is
In which of the following hydrolysis (complete) reaction is not released?
When and in ratio is heated at in a sealed nickel vessel it form?
How many right angles are there in
upon reaction with form compound . Which is true for compound
The shape of is
Structure of is correctly represented by
In xenon fluorides most reactive in and is
Which one of the following does not form during the hydrolysis of
Pick out the correct statement for
Match the items of Columns I and II and mark the correct option.
| Column I | Column II |
| (A) Its partial hydrolysis does not change oxidation state of central atom | (1) |
| (B) It is used in modern diving apparatus | (2) |
| (C) It is used to provide inert atmosphere for filling electrical bulbs | (3) |
| (D) Its central atom is in hybridisation | (4) |
The shape of is
on partial hydrolysis with water, produces a compound The same compound is formed when reacts with silica. The compound is:
It is unfeasible for which of the following reactions of Xenon compounds to take place?
In which one of the following pairs, the second compound has more number of bonds than the first one?
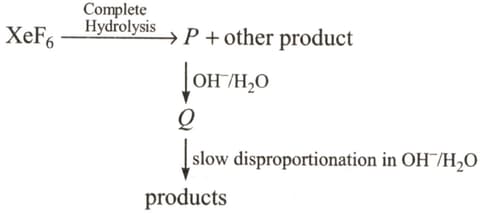
Under ambient conditions, the total number of gases released as products in the final step of the reaction scheme shown below is: