Oxoacids of Chlorine: Preparation, Structure, Properties and Uses
Oxoacids of Chlorine: Preparation, Structure, Properties and Uses: Overview
This Topic covers sub-topics such as Oxoacids of Halogens and Structures of Oxoacids of Chlorine
Important Questions on Oxoacids of Chlorine: Preparation, Structure, Properties and Uses
What products are expected from the disproportionation reaction of hypochlorous acid?
The oxidizing power of oxyacids of chlorine depend upon
Why does fluorine form only one oxoacid, . Explain.
Identify the chemical formula of oxoacid of halogen, chloric acid.
The correct order of acidity of oxoacids of chlorine is
Identify the name of oxoacid of halogen, .
Explain why fluorine does not form oxoacids?
Which oxoacid of chlorine is the most acidic?
Arrange the following :
Increasing order of thermal stability
Increasing acid strength
Increasing reducing nature
Increasing oxidation number of iodine
Increasing acid strength
Increasing oxidising power
Increasing acid strength
Increasing electronegativity
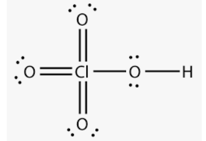
Above structure is of _____ acid.
The structure of is:
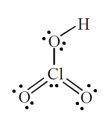
.
Which of the following oxyacid of chlorine has highest thermal stability?
Which are the different oxyacids of halogens?
The correct order of acidic strength of following halo acids is-
I.
II.
III.
Among the following, which has maximum acidic character?
The correct order of acidic strength is:
Among the following oxoacids, the strongest oxidizing agent is
Strongest conjugate base is
Strongest conjugate base is
How can a central iodine be bonded in octahedral ortho periodic acid?