Methods of Preparation of Alcohols
Methods of Preparation of Alcohols: Overview
This Topic covers sub-topics such as Methods of Preparation of Alcohols, Mechanism of Acid Catalysed Hydration of Alkenes, Preparation of Alcohols by Reduction of Aldehydes and Ketones and, Preparation of Alcohols By Acid Catalysed Hydration of Alkenes
Important Questions on Methods of Preparation of Alcohols
Match the conversions with the reagents that are used in the process:
| i. | Phenol to benzoquinone | a. | H2O/H+ |
| ii. | Propanone to 2-methylpropan-2-ol | b. | Na2Cr2O7/H2SO4 |
| iii. | Propene to propan-2-ol | c. | CH3MgBr/H2O |
An organic compound A on reduction forms compound B . B reacts with HBr to form the compound C. C with Mg forms Grignard reagent D which reacts with A to form a product which on hydrolysis gives E. Identify A to E.
In the following sequence of reactions,
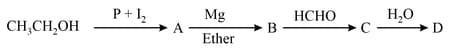
The compound D is –
Which of the following compound gives -Ethylpentan--ol by the action of ethyl magnesium iodide followed by acid hydrolysis?
Which of the following alcohols cannot be prepared by reduction of carbonyl compounds?
In the conversion of starch into alcohol, the following enzymes are used:
Propene on oxidation with diborane in presence of alkaline hydrogen peroxide gives _______.
The major products and in the following reaction sequence, are
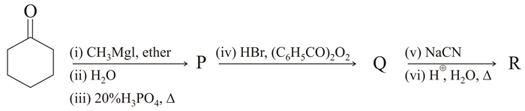
, Identify product is :
Five isomeric p-substituted aromatic compound (A) to (E) with molecular formula C8H8O2 are given for identification. Based on the following observation, give the structures of the compounds.
i. Both (A) and (B) form a silver mirror with Tollen's reagent. (B) also gives a positive test with neutral FeCl3 solution.
ii. (C) gives positive iodoform test.
iii. (D) is readily extracted in aqueous NaHCO3 solution.
iv. (E) on acid hydrolysis gives 1, 4-dihydroxybenzene.
Compound (E) is :
Which of the following are used to convert into?
The reduction of acetone by gives:
Through which of the following reactions number of carbon atoms can be increased in the chain?
Using Grignard reagent, suggest synthesis of following alcohol from aldehydes or ketones. Whenever possible, suggest more than one combination.
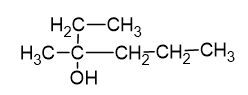
Using Grignard reagent, suggest synthesis of following alcohol from aldehydes or ketones. Whenever possible, suggest more than one combination.
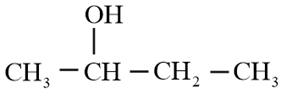
Using Grignard reagent, suggest synthesis of following alcohol from aldehydes or ketones. Whenever possible, suggest more than one combination.
Using Grignard reagent, suggest synthesis of following alcohol from aldehyde or ketone. Whenever possible, suggest more than one combination.
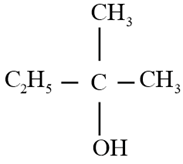
Write the structure of carbonyl compound that can be converted by reduction methods into following alcohol.
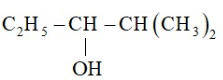
Write the structure of carbonyl compound that can be converted by reduction method into following alcohol.