Preparation of Aldehydes and Ketones
Preparation of Aldehydes and Ketones: Overview
This Topic covers sub-topics such as Stephen Reaction, Oxo Process, Methods of Preparation of Aldehydes and Ketones, Preparation of Aldehydes and Ketones by Oxidation of Alcohols, Methods of Preparation of Aldehydes and, Methods of Preparation of Ketones
Important Questions on Preparation of Aldehydes and Ketones
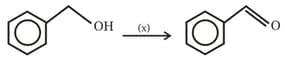
Reagent (x) is
The reagents used for the following conversions are:
cyclohexanol to cyclohexanone
bromobenzene to benzoic acid
Which of the following on heating with aqueous KOH, produces acetaldehyde?
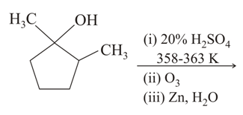
methylcyclohexadiene on reductive ozonolysis with yields products A and B.The total number of hybridized carbons in A and B are
How the alkenes are the substrate and are the reagent of oxo process.
Write the oxo process to produce aldehydes?
Write a chemical reaction for the oxo process.
Explain the oxo process.
Oxo process is an industrial process to prepare the ketone.
On hydrolysis in the presence of acid, nitroalkane gives
Secondary nitro alkane gives _____ on hydrolysis with hydrochloric acid.
Give one method of preparation of aldehydes.
Complete the following reaction:
Which of the following is not synthesized by Rosenmund reduction:
Predict the product of the following conversion:
Ethyl methyl ketone is obtained by heating calcium salts of
Benzene reacts with acetyl chloride in the presence of anhydrous , we get Aceto_____ as the product.
The major product(s) in the following reactions is/are
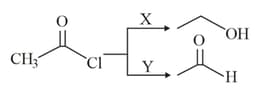
and are respectively :
A number of reactions in which the final product is an aldehyde. Find out .
