Cleansing Agents
Cleansing Agents: Overview
This topic covers concepts, such as Cleansing Agents, Types of Cleansing Agents, Soaps, Types of Soaps, Medicated Soaps, Toilet Soaps, Shaving Soaps, Laundry Soaps, Problem with Soaps in Hard Water, and Synthetic Detergents.
Important Questions on Cleansing Agents
Match the following:
| i. | Cationic detergents | a. | Group of biomolecules catalyse the reaction |
| ii. | Enzymes | b. | Aspartame |
| iii. | Sweetening agents | c. | Cetyltrimethylammonium chloride |
Among the following, an example of soap is
In order to prevent rapid drying, shaving soaps contains
Main chemical components of 'Dettol' are
An anionic detergent with formula is known as
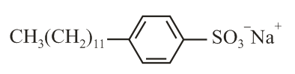
Biodegradable detergent should have
Which is correctly matched regarding the use?
Glyceryl oleate can be represented by the formula
Which of the following is incorrect?
Which type of detergents are preferably used in liquid dish washing?
Soaps on reaction with hard water forms insoluble scum due to the formation of
Soap powders and scouring soap contains builders like
The fillers that can be present in laundry soap is/are
Soaps which are made by dissolving the soap in ethanol and then evaporating the excess solvent
Glycerylester of Stearic acid Sodium stearate
Product in the above reaction is
Soaps are sodium or potassium salt of long chain fatty acids like
The correct structure of Bithional is
Which of the following is a by-product of soap-industry?
Which of the following is not an example of detergent?
Which of the following is a biodegradable detergent?
