Electrode Potential and Standard Electrode Potential
Electrode Potential and Standard Electrode Potential: Overview
This Topic covers sub-topics such as Electrode Potential, Standard Hydrogen Electrode, Reference Electrodes, Measurement of EMF of a Cell, Reduction Electrode Potential, Standard Reduction Electrode Potential and, Oxidation Electrode Potential
Important Questions on Electrode Potential and Standard Electrode Potential
A standard hydrogen electrode has zero electrode potential because
When the samples of copper with zinc impurity is to be purified by electrolysis, the appropriate electrodes are –
The electode potential of anode at standard condition is called standard _____ potential.
The standard oxidation potential of zinc is _____ .
The difference between the electrode potentials of two electrodes when no current is drawn through the cell is called _____.(Cell emf/Cell potential)
The reaction is spontaneous if the cell potential is _____.(Positive/Negative)
EMF of a cell in terms of reduction pontential of its left and right electrodes is:
The reaction is spontaneous if the cell potential is
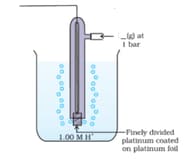
The IUPAC name of the gas released in the above diagram, where a blank is given is :
(Note : Fill the blanks by writing the chemical formulas in straightforward manner without using subscript. For example, the chemical formula of methane should be filled in the blank as and not as )
A reference electrode refers to an electrode that has a variable electrode potential.
The following reaction occurs in a galvanic cell . If and the standard cell potential will be
The standard potential for the cell reaction,
where is
Can we keep solution in a copper container ?
Which cell will measure standard electrode potential of copper electrode?
Which cell will measure standard electrode potential of copper electrode?
Write balanced equations for the half-reaction and calculate the reduction potential at for the following half cell:
.
Consider the following values and half reactions:
.
The half reactions can be used to construct three galvanic cells. Which will have the highest cell potential?
What are standard electrode potential and standard emf of the galvanic cell?
What conditions are required for a cell potential to be called standard cell potential?
Define the terms: oxidation potential, reduction potential and cell potential.