Close Packed Structures and Packing Efficiency
Close Packed Structures and Packing Efficiency: Overview
This topic covers concepts, such as, Relation between Edge Length and Radius of a Constituting Atom for a BCC Structure & Relation between Edge Length and Radius of a Constituting Atom for a Simple Cubic Structure etc.
Important Questions on Close Packed Structures and Packing Efficiency
Silver crystallises with face-centred cubic unit cells and each side of the unit cell has a length of . Determine the radius of an atom of silver. (Assume that each face atom is touching the four corner atoms.)
A metallic element crystallises into lattice having a layering sequence of Any packing of sphere leaves out voids in the lattice. Determine what percentage by volume of this lattice is empty space.
Sodium metal crystallises in a body centred cubic lattice with the cell edge, What is the radius of a sodium atom?
has bcc structure with edge length . The shortest inter ionic distance in between and is:
The distance between an octahedral and a tetrahedral void in in FCC lattice would be:
(Consider edge length is )
What is the radius of sodium atom if it crystallizes in structure with the cell edge of ?
| Column I (Type of crystal) | Column II (Location of cations/anions) |
| (A) | (p) Cations-fcc, Anions-all tetrahedral voids |
| (B) | (q) Anions-fcc, Cations-all tetrahedral voids |
| (C) | (r) Anions-fcc, Cations-all octahedral voids |
| (D) | (s) Anions-fcc, Cations-alternate tetrahedral voids |
The relationship between edge length and the radius of the simple cubic unit cell is _____.
What is coordination number? Give the coordination number of the simple cubic lattice.
The coordination number for molybdenum in hexagonal closed packing is
The coordination number in one-dimension close packing is
The coordination number is equal to _____ in one-dimensional close packing in crystalline solid.
Describe close packing in one dimension in crystalline solids.
The lattice generated in three-dimensional closed packing from two-dimensional square close-packed layers is
Define three-dimensional close packing from two-dimensional square close-packed layers.
Two dimensions coordination number of a molecule in square close-packed layers is
Discuss the alignment of spheres in the square closed packing in two dimensions.
Octahedral voids are formed when the triangular voids in second layer exactly overlap with similar voids in the first layer.
A unit cell of (fluorite structure) is made up of eight ions and four ions.
A two dimensional solid is made by alternating circles with radius and such that the sides of the circles touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length and
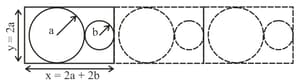
The ratio for which the packing fraction is minimized is closest to:
