Tests for Lead(II) Ion
Tests for Lead(II) Ion: Overview
This Topic covers sub-topics such as Analysis of Group-I Cationic Radicals, Sodium Hydroxide Solution Test for Lead(II) Ion, Tests for Lead(II) Ion, Hydrogen Sulphide Test for Lead(II) Ion and, Tests for Lead(II) Ion in Group-II A
Important Questions on Tests for Lead(II) Ion
Formation of which complex, among the following, is not a confirmatory test of ions
Which one doesn't gives test?
The reaction of and in water produces a precipitate that dissolves upon the addition of of appropriate concentration. The dissolution of the precipitate is due to the formation of
Find the number of compounds which have yellow colour precipitate from the given compounds:
Find the number of compounds which have yellow colour precipitate from the given compounds:
Balance the chemical equation
Write the chemical reaction of the product formed by reaction of with hydrogen sulphide in acidic medium.
_____ and _____ ions give white precipitates with solution which are soluble in excess of the reagent.
Name the probable cation present in the following observation:
White precipitate insoluble in but soluble in .
Addition of to salt gives a precipitate. The colour of precipitate is
Which of the following is incorrectly matched ?
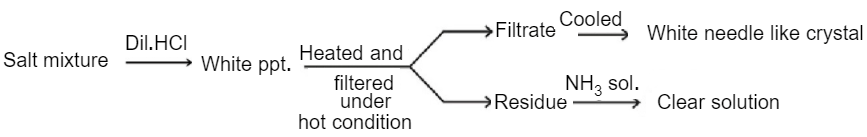
Read the following sequence of reactions:
What are the compounds and ?
The compound which will turn black colour on adding to it is
Which of the following metal ions does not form a complex with ammonia?
What are the cations present in the salt ?
Consider a colorless salt (X) which is soluble in water and also in alcohol and amines. Iron (II) sulphate can easily reduce a solution of (X). (X) gives a brown gas (Y) and a grey residue on strong heating . (X) dissolves in ammonia to give a solution (Z) which gives silver mirror with aldehydes. A solution of (X) also reacts with potassium chromate solution to gives a brick red precipitate. Choose the correct option :
Choose the correct option in which the compound gives a precipitate with but not with .
Among these compounds, the ones that are either sparingly soluble or insoluble in solution are:
Which of the following combinations in an aqueous medium will give a yellow ppt.?
Cotyledons are also called-
