First Law of Thermodynamics
First Law of Thermodynamics: Overview
This topic covers concepts, such as Sign Convention in Thermodynamics, First Law of Thermodynamics and Equation for Work Done.
Important Questions on First Law of Thermodynamics
Given below are two statements:
Statement I: If heat is added to a system, its temperature must increase.
Statement II: If positive work is done by a system in a thermodynamic process, its volume must increase.
In the light of the above statements, choose the correct answer from the options given below
A source supplies heat to a system at the rate of If the system performs work at a rate of The rate at which internal energy of the system increases is
The amount of heat supplied to a gas in a system is equal to , the system in return does of work on the surrounding. Find change in internal energy of the gas.
If amount of heat given to the system be Joules and the amount of work done by the system is . If initial internal energy of the system is . Final internal energy of the system is
ideal gas expands from volume to,. This may be achieved by either of the three processes: isobaric, isothermal and adiabatic. Let be the change in internal energy of the gas, be the quantity of heat added to the system and W be the work done by the gas. Identify which of the following statements is false for?
of water at atmospheric pressure has volume of and when boiled it becomes of steam. The heat of vaporization of water is . Then the change in its internal energy in this process is
An ideal gas is expanded adiabatically at an initial temperature of so that its volume is doubled. The final temperature of the hydrogen gas is
In an insulated container of water is stirred with a rod to increase the temperature. Which of the following is true?
of water is heated from to , if its volume remains constant, then the change in internal energy is (specific heat of water )
of liquid water at undergoes a phase change into steam at at atm (take it to be ). The initial volume of the liquid water was which is changed to of steam. Find the change in the internal energy of the system.
[Use heat of vaporization ]
In the diagram shown and cal. If cal for the curved path value of for path , will be
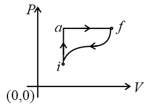
Which of the following is the correct equation?
A cube of side made of iron and having a mass of is heated from to . The specific heat for iron is and the coefficient of volume expansion is , the change in the internal energy of the cube is (atm pressure )
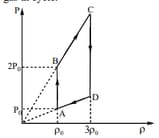
moles of an ideal gas is taken through a cyclic process . Pressure - density diagram of which is shown in adjacent figure. If molar mass of gas is , what will be the work done by gas in cycle.
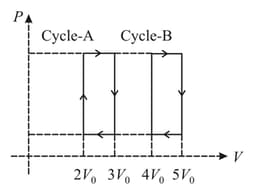
A heat engine used one mole of an ideal monatomic gas as a working substance. The engine can follow either cycle or cycle in the diagram. The ratio of the efficiencies of the cycles is If the value of is Find the value of
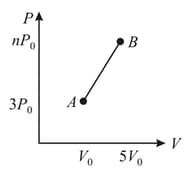
One mole of a monoatomic ideal gas follows a process as shown and The average molar heat capacity for the process is The value of on axis (see following figure) is
A cylindrical vessel is divided in two parts by a fixed partition which is perfectly heat conducting. The wall and piston are thermally insulated from surroundings. The left side contains moles of gas with at temperature of The right side contains moles of a mixture of gases with at same temperature of The piston compresses slowly the right side from volume of to Find the total change in internal energy of gases. (take unit and consider ideal gases only)
According to the first law of thermodynamics, In special cases the statement can be expressed in different ways. Which of the following is not a correct expression?
An ideal gas is allowed to expand freely against vacuum in a rigid insulated container. The gas undergoes
When heat is supplied to the system then is ____ and is ____ when heat is extracted from the system. Choose the appropriate option.
